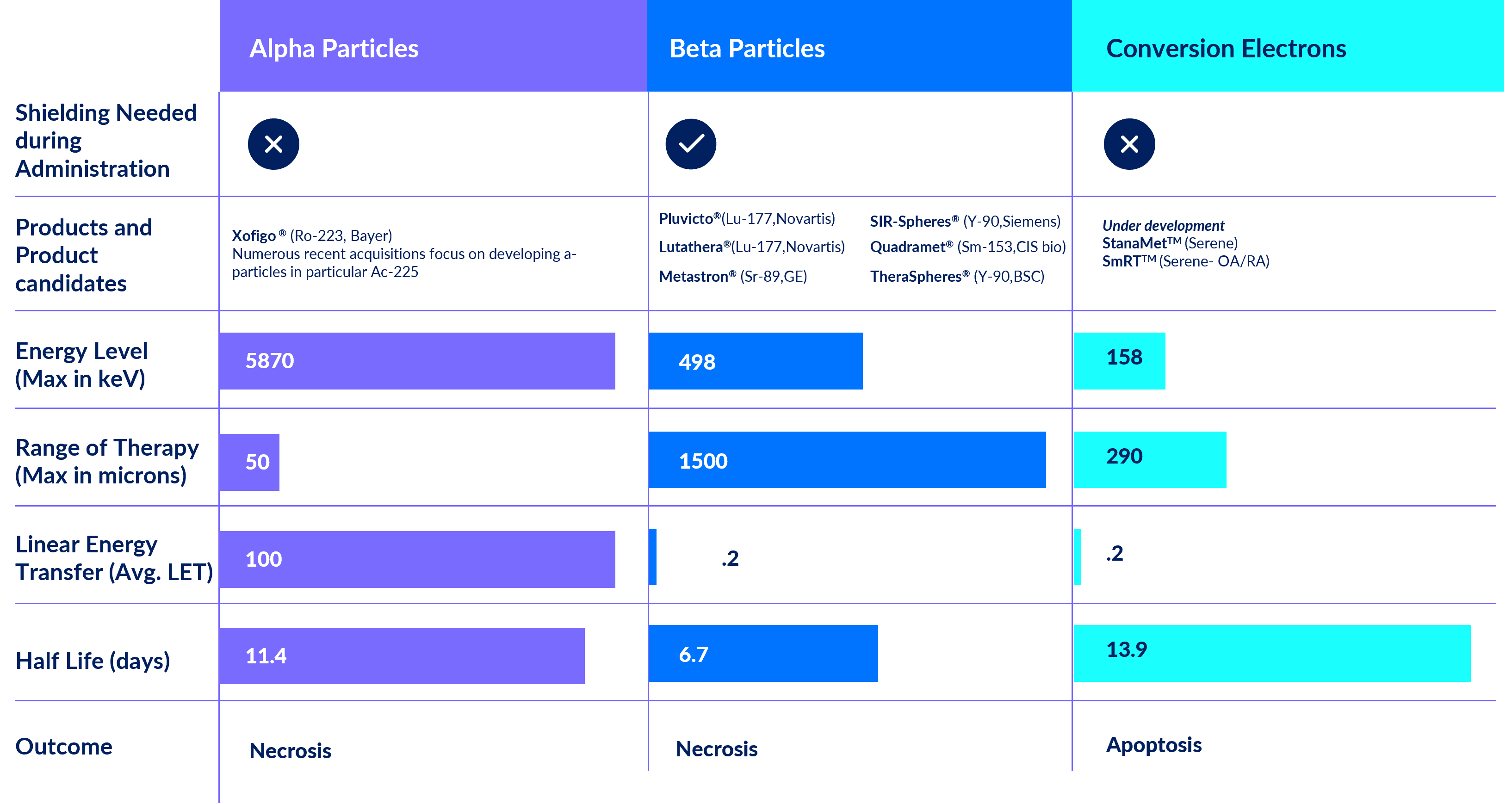
Tin-117m is an isotope that emits therapeutic conversion electrons
that deposit their energy completely out to ~300μm in tissue, and photons that can be imaged with a standard gamma camera.
This unique conversion electron energy has several distinct advantages over traditional radiation therapy including an ideal two-week half-life and an unprecedented safety profile which allows shipping with minimal handling procedures.
Tin-117m is manufactured at-will in the United States and is available to investigators for research purposes. Development of cGMP manufacturing for pre-clinical and clinical studies as well as for commercial products has been achieved for several products. There are no other isotopes identified that have the unique characteristics of tin-117m, and this makes it ideal for these medical applications.
Tin-117m has been conjugated with small and large molecules as well as with biologic molecules.
Electroplating of tin-117m to stainless steel has been accomplished, and is suitable for medical devices.
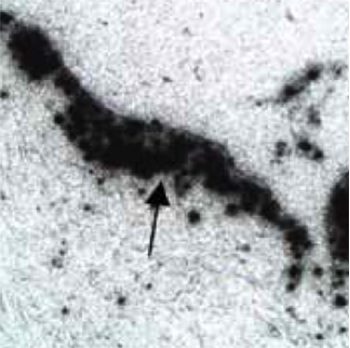
Proprietary Homogeneous Tin-117m Colloid which can be used for multiple indications is consistently manufactured to a specific, reproducible size. This particle size, when injected intraarticular, is large enough to allow the tin-117m colloid to remain >99% within the joint, and yet is small enough to be readily engulfed by synovial macrophages, as seen in this autoradiogram showing colloid particles dispersed throughout the synovium of a joint (arrow head). Radiosynoviorthesis is the procedure whereby a radioisotope, typically in colloidal form, is injected into the joint space of an inflamed joint, ultimately decreasing the pain associated with the inflammatory condition. The procedure has been used in humans for decades to treat osteoarthritis, rheumatoid arthritis and other conditions outside of the United States using β particle-emitting radioisotopes. Existing β particle synovial treatments can be highly successful but the radioisotopes used have negative consequences when their energy is deposited beyond the tissues of the joint space. These negative issues with other isotopes have been resolved with tin-117m colloid.
Other Serene Development Projects–Serene has several active, advanced tin-117m-based projects:
- Cardiovascular–Vulnerable plaque is the primary target for tin-117m linked to annexin V. The first indication, which has successfully completed a Phase 1/2 human trial, is for the imaging and early treatment of symptomatic, inoperable high-grade carotid artery stenosis.
- Oncology–For the treatment of cancer indications, several therapeutic molecules and devices are being developed including a clinical trial for bone metastases and development of Sn-117m-microparticles for HCC. Several tin-117m labeled molecules for other oncologic indications are also in the pipeline. Additionally, tin-117m electroplated devices for cancer treatment and palliation are in development.
Manufacturing
Tin-117m isotope is routinely produced for Serene at reactor and cyclotron sites around the world, enabling the production of a range of medical products based on this isotope. Commercial and clinical products are currently manufactured at several cGMP sites in the United States.
